Zirconocene-catalyzed direct (trans)esterification of acyl acids (esters) and alcohols in a strict 1 : 1 ratio under solvent-free conditions†
Abstract
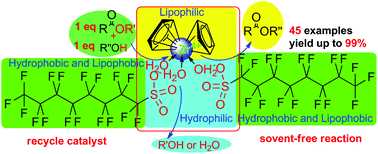
A highly efficient way for the direct (trans)esterification of acyl acids (esters) and alcohols in a strict 1 : 1 ratio using a zirconocene complex (1, 1 mol%), a strong Lewis acid of good water tolerance, as a catalyst under solvent-free conditions has been developed. A wide range of acid and alcohol (esters) substrates undergo (trans)esterification to produce carboxylic ester motifs in moderate to good or excellent yields with good functional tolerance, such as that towards C–Br as well as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds. And complex 1 can be recycled six times without showing a significant decline in catalytic efficiency. It was demonstrated that cyclandelate, which is used to treat high blood pressure as well as heart and blood-vessel diseases, can be directly synthesized on a gram scale with 81% yield (6.70 g) using complex 1.
C bonds. And complex 1 can be recycled six times without showing a significant decline in catalytic efficiency. It was demonstrated that cyclandelate, which is used to treat high blood pressure as well as heart and blood-vessel diseases, can be directly synthesized on a gram scale with 81% yield (6.70 g) using complex 1.
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...