Facile preparation of bio-based polyesters from furandicarboxylic acid and long chain diols via asymmetric monomer strategy†
Abstract
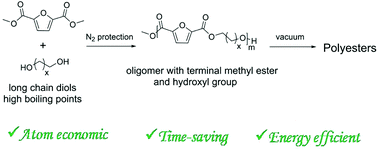
In this study, we propose a novel strategy for the preparation of furandicarboxylic acid (FDCA) based polyesters through the melt polycondensation of asymmetric monomers with terminal methyl ester and hydroxyl groups. A series of bio-based polyesters were prepared from dimethyl 2,5-furandicarboxylate and diols containing long alkyl chains. In this strategy, volatile methanol rather than diols with high boiling points was removed as a by-product. The effects of the catalyst concentration, reaction temperature and reaction time on the molecular weight and PDI were investigated. Polyesters with high molecular weights and without coloration were obtained at a lower polycondensation temperature and in less time in comparison with previous methods. The thermal and mechanical properties of the as-prepared polyesters were investigated by DSC, TGA, DMA and tensile testing. The results revealed that the thermal and mechanical properties of the polyesters including Tm, Tc, Tg, Young's modulus, tensile strength and elongation at break, not only depended on the number of methylene groups but also were related to the odd–even effect.


 Please wait while we load your content...
Please wait while we load your content...