An environmentally benign and selective electrochemical oxidation of sulfides and thiols in a continuous-flow microreactor†
Abstract
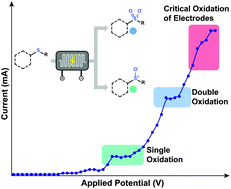
A practical and environmentally benign electrochemical oxidation of thioethers and thiols in a commercially-available continuous-flow microreactor is presented. Water is used as the source of oxygen to enable the oxidation process. The oxidation reaction utilizes the same reagents in all scenarios and the selectivity is solely governed by the applied potential. The procedure exhibits a broad scope and good functional group compatibility providing access to various sulfoxides (15 examples), sulfones (15 examples) and disulfides (6 examples). The use of continuous flow allows the optimal reaction parameters (e.g. residence time, applied voltage) to be rapidly assessed, to avoid mass- and heat-transfer limitations and to scale the electrochemistry.
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...