Photoorganocatalytic synthesis of lactones via a selective C–H activation–alkylation of alcohols†
Abstract
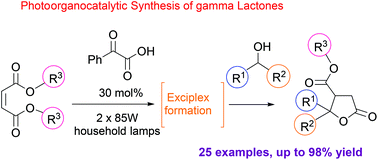
Selective C–H activation is an area of growing importance in modern organic chemistry. Herein, we report our efforts in combining organocatalysis and photocatalysis for the development of a highly efficient and selective visible-light mediated protocol for the C–H activation and addition of various alcohols to a plethora of Michael acceptors, followed by a cyclization reaction leading to lactones, a repeatedly occurring motif in nature. Utilizing phenylglyoxylic acid as the photocatalyst and common household bulbs as the light source, we describe a versatile α-alkylation/lactonization of alcohols with α,β-unsaturated esters leading to products in excellent yields. The reaction mechanism was extensively studied.


 Please wait while we load your content...
Please wait while we load your content...