Synthesis of ethanol from paraformaldehyde, CO2 and H2†
Abstract
Ethanol is an important bulk chemical and alternative fuel that is currently synthesized by catalytic hydration of ethylene or fermentation of foods. CO2 is a cheap and renewable carbon resource. Transformation of CO2 into useful chemicals is an interesting topic in green chemistry. Production of ethanol using CO2 and H2 is a promising route, but the efficiency of the reaction is not satisfactory. In this paper, we propose a protocol to synthesize ethanol from paraformaldehyde, CO2 and H2. The reaction could be efficiently accelerated by a Ru–Co bimetallic catalyst using LiI as the promoter in 1,3-dimethyl-2-imidazolidinone (DMI) under mild conditions. The selectivity of ethanol in total products reached 50.9 C-mol%, which was obviously higher than that of the reported routes. Furthermore, the TOF of ethanol based on Ru metal was as high as 17.9 h−1. To our knowledge, this is the first report on ethanol synthesis from paraformaldehyde, CO2 and H2. A detailed study indicated that the outstanding results of the reaction originated from the synergy of paraformaldehyde hydrogenation, reverse water gas shift reaction and methanol homologation.
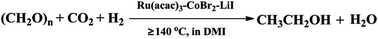


 Please wait while we load your content...
Please wait while we load your content...