Ecocatalyzed Suzuki cross coupling of heteroaryl compounds†
Abstract
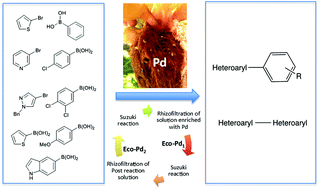
A bio-based EcoPd was developed for the Suzuki cross coupling of heteroaryl compounds. Based on the ability of Eichhornia crassipes to bioconcentrate Pd in its roots, we addressed the transformation of plant-derived Pd metals to green catalysts. The methodology is based on eco-friendly procedures. It allowed the preparation of a wide range of heterocyclic biaryl and heterocyclic–heterocyclic biaryl compounds, with a low Pd catalyst loading. EcoPd was found to have the ideal microstructure to promote complex Suzuki reactions without ligands or additives. For the first time, post-reaction solution was treated by rhizofiltration. The resulting EcoPd has been reused with the same performance. This work has established the ecocatalysis concept as a powerful strategy for Pd sustainability, with the development of homogeneous catalysts that are easily recycled and reused.
- This article is part of the themed collection: International Symposium on Green Chemistry 2017


 Please wait while we load your content...
Please wait while we load your content...