Synthesis of 3-alkoxypropan-1,2-diols from glycidol: experimental and theoretical studies for the optimization of the synthesis of glycerol derived solvents†
Abstract
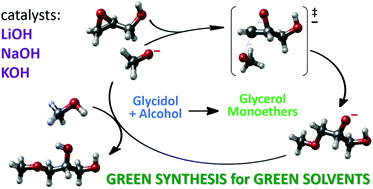
A straightforward synthetic methodology has been derived for the synthesis of glycerol monoethers from glycidol and alcohols. Several homogeneous and heterogeneous basic catalysts have been tested, the best results being obtained with readily available and inexpensive alkaline metal hydroxides. In the best case, good yield of the desired monoether is obtained under smooth reaction conditions, always with total conversion of glycidol. The selectivity of the reactions mainly depends on the alcohol used, due to the concurrence of undesired side reactions. A mechanistic study carried out through computational DFT calculations, in which solvent effects are taken into account, also complemented the experiments, has allowed to identify the main reaction paths taking place under reaction conditions, giving insights into the main causes affecting the reaction selectivity and also into how it could be improved.


 Please wait while we load your content...
Please wait while we load your content...