β-Amino acid derived gemini surfactants from diformylfuran (DFF) with particularly low critical micelle concentration (CMC)†
Abstract
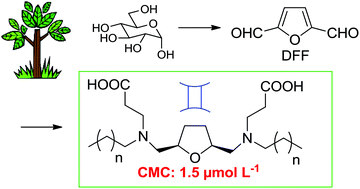
Starting from diformylfuran (DFF) obtained from biomass, a new family of gemini surfactants has been synthesized. The polar group is composed of two amphoteric amino acids attached to a tetrahydrofuran ring. During the preparation, the formation of metal salts is avoided in the final steps. At very low critical micelle concentrations (CMCs) of around 1.5 μmol L−1, an efficient decrease of the surface tension of up to 30 mN m−1 is measured. Below the CMC, the surface excess Γ varies with the surfactant bulk concentration. In a concentration interval below the CMC, a linear relationship between Γ and the bulk concentration is observed. Wetting properties are reported and bactericidal activities have been detected. Efficient antifungal activity against Fusarium graminearum has been detected.
- This article is part of the themed collection: International Symposium on Green Chemistry 2017


 Please wait while we load your content...
Please wait while we load your content...