An environmentally benign double Michael addition reaction of heterocyclic ketene aminals with quinone monoketals for diastereoselective synthesis of highly functionalized morphan derivatives in water†
Abstract
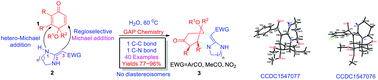
A efficient and concise one-pot procedure has been developed for the synthesis of highly functional morphan derivatives 3 based on diastereoselective double Michael addition reactions of various quinone monoketals 1 with a variety of heterocyclic ketene aminals (HKAs) 2 in the green solvent water at 60 °C. This protocol is especially suitable for efficient and rapid parallel syntheses of N-containing bridged heterocycles possessing pharmacological activities. As a result, a library of highly functional morphan derivatives was easily synthesized using this reported environmentally benign, mild, and catalyst-free one-pot reaction.


 Please wait while we load your content...
Please wait while we load your content...