A straightforward one-pot synthesis of bioactive N-aryl oxazolidin-2-ones via a highly efficient Fe3O4@SiO2-supported acetate-based butylimidazolium ionic liquid nanocatalyst under metal- and solvent-free conditions†
Abstract
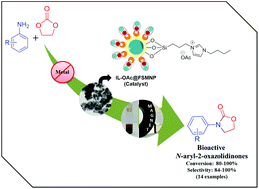
In the present study, we report the fabrication and characterization of novel acetate-based butylimidazolium ionic liquid immobilized silica-coated magnetic nanoparticles (IL-OAc@FSMNP). The synthesized nanocomposite proves its supremacy as an environmentally benign catalyst in the reaction of aniline and its derivatives with ethylene carbonate to form bioactive N-aryl oxazolidin-2-ones under metal-, ligand-, and solvent-free conditions. The catalyst offers excellent assemblies of hydrogen-bond donors and acceptors, which activate the substrates, thereby delivering good-to-excellent product yields with a conversion and selectivity of more than 99%. Additionally, mild reaction conditions, wide substrate scope, effortless catalytic recovery and recyclability of the catalyst up to eight consecutive cycles offer the potential for scale-up in various pharmaceutical applications.


 Please wait while we load your content...
Please wait while we load your content...