Selective palladium-catalysed synthesis of diesters: alkoxycarbonylation of a CO2-butadiene derived δ-lactone†
Abstract
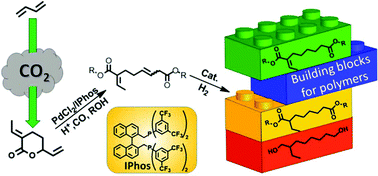
Novel unsaturated C10 diesters are produced via the alkoxycarbonylation of δ-lactone 1 (3-ethylidene-6-vinyltetrahydro-2H-pyran-2-one), derived from the telomerization of CO2 and butadiene. The key for the selective valorization of 1 is the use of a catalytic system based on PdCl2, a chelating phosphine bearing electron-withdrawing groups and an acidic promoter. The unsaturated C10 methyl diester can be easily hydrogenated on Pd/C under mild conditions to afford its corresponding saturated diester. Subsequent hydrogenation using the homogeneous [Ru(acac)3]/Triphos catalysts gives 2-ethyloctane-1,8-diol in high yield. The overall procedure allows synthesizing new building blocks for the manufacturing of renewable polymers and polymer processing materials.


 Please wait while we load your content...
Please wait while we load your content...