The formation of p-toluic acid from coumalic acid: a reaction network analysis†
Abstract
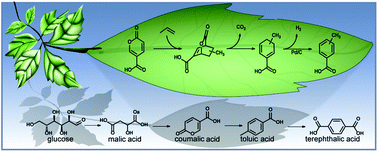
Diels–Alder cycloaddition of biomass-derived 2-pyrone coumalic acid (CMA) with propylene provides an alternative pathway to produce toluic acid (TA), a precursor to terephthalic acid (TPA) which is a key component in the manufacture of polyethylene terephthalate (PET). To maximize yield and selectivity of the preferred isomer p-toluic acid (p-TA) we herein report a detailed reaction network that was established through investigating the influence of the dehydrogenation catalyst, solvent, temperature and reaction time on the formation of p-/m-TA. We further provide experimental results showing that the inverse electron demand Diels–Alder (IEDDA)/decarboxylation cascade of the reaction of CMA with propylene was not influenced by the catalyst, and, therefore, was solely dependent on temperature. Evident from changes in the p-/m-ratio of TA decreasing from 6.91 to 4.43 with increasing temperature and reaction time, p-TA was both the thermodynamic and kinetically favoured product. TA formation from CMA and propylene afforded an overall yield of >85 mol%, using γ-valerolactone (GVL) as the primary solvent. Throughout the reaction network analysis we observed that the TA intermediates 3-/4-methylcyclohexa-1,5-diene carboxylic acid were reactive towards by-product formation whereas TA was quite stable under reaction conditions. Henceforth, rapid dehydrogenation of the diene intermediates is crucial to maximize selectivity of the desired aromatic product. Lastly, CMA thermally decomposes under reaction conditions which was rapidly accelerated by having as low as 5 vol% water in the solvent, demonstrating the importance of water-free solvent to limit CMA decomposition and maximize product yield.


 Please wait while we load your content...
Please wait while we load your content...