Development of a lipase-mediated epoxidation process for monoterpenes in choline chloride-based deep eutectic solvents†
Abstract
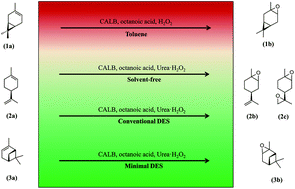
Chemical syntheses in contemporary process industries today are predominantly conducted using organic solvents, which are potentially hazardous to humans and the environment alike. Green chemistry was developed as a means to overcome this hazard and it also holds enormous potential for designing clean, safe and sustainable processes. The present work incorporates the concepts of green chemistry in its design of a lipase-mediated epoxidation process for monoterpenes; the process uses alternative reaction media, namely deep eutectic solvents (DESs), which have not been reported for such an application before. Choline chloride (ChCl), in combination with a variety of hydrogen bond donors (HBD) at certain molar ratios, was screened and tested for this purpose. The process was optimized through the design of experiments (DoE) using the Taguchi method for four controllable parameters (temperature, enzyme amount, peroxide amount and type of substrate) and one uncontrollable parameter (DES reaction media) in a crossed-array design. Two distinct DESs, namely glycerol : choline chloride (GlCh) and sorbitol : choline chloride (SoCh), were found to be the best systems and they resulted in a complete conversion of the substrates within 8 h. Impurities (esters) were found to form in both the DESs, which was a concern; as such, we developed a novel minimal DES system that incorporated a co-substrate into the DES so that this issue could be overcome. The minimal DES consisted of urea·H2O2 (U·H2O2) and ChCl and exhibited better results than both the GlCh and SoCh systems; complete conversions were achieved within 2 h for 3-carene and within 3 h for both limonene and α-pinene. Product isolation with a simple water/ethyl acetate based procedure gave isolated yields of 87.2 ± 2.4%, 77.0 ± 5.0% and 84.6 ± 3.7% for 3-carene, limonene and α-pinene respectively.


 Please wait while we load your content...
Please wait while we load your content...