Hydrolytic hydrogenation of chitin to amino sugar alcohol†
Abstract
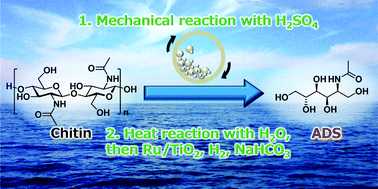
Chitin is the second most abundant biomass and characteristically contains nitrogen atoms in its monomer units. These favourable features promote chitin to be a potential resource for renewable organonitrogen compounds. 2-Acetamido-2-deoxysorbitol (ADS) is an attractive target in the derivatives of chitin, but the conversion of chitin to ADS has not been reported so far. In this work, we demonstrate the catalytic conversion of chitin to ADS using mechanocatalysis in the presence of H2SO4 and subsequent hydrolytic hydrogenation by H2SO4 and Ru/TiO2 without any purification process. Our study clarified that the yield of ADS is strongly influenced by the reaction temperature and pH. The hydrolysis favourably proceeds at high temperature and low pH (2.0), but the hydrogenation needs a low temperature and a specific pH of 3–4 to achieve high selectivity. Specifically, in the hydrogenation step, an acid causes various side-reactions of amide and hemiacetal groups especially in the presence of a Ru catalyst, whereas even a small amount of base drastically accelerates the retro-aldol reaction to form erythritol and N-acetylethanolamine. Therefore, a one-pot but two-step reaction is necessary to optimise both the hydrolysis and hydrogenation steps and maximise the overall yield of ADS up to 52%.


 Please wait while we load your content...
Please wait while we load your content...