A scalable carboxylation route to furan-2,5-dicarboxylic acid†
Abstract
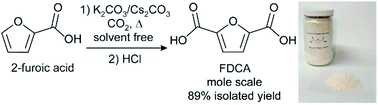
Furan-2,5-dicarboxylic acid (FDCA) is a biomass-derived diacid that can be used to make polymers including polyethylene furandicarboxylate (PEF), a highly attractive substitute for petroleum-derived polyethylene terephthalate (PET). Current FDCA syntheses require edible fructose as the feedstock, entail a difficult oxidation step that generates undesirable aldehyde impurities, and have moderate yields. As an alternative, carbonate-promoted C–H carboxylation enables the synthesis of FDCA from 2-furoic acid and CO2. This route is potentially advantageous because 2-furoic acid is made from furfural, a feedstock produced commercially from inedible lignocellulosic biomass, and it obviates late-stage oxidation. In the carboxylation reaction, salt mixtures composed of alkali furan-2-carboxylate (furoate) and alkali carbonate (M2CO3) are heated under CO2 in the absence of solvent or catalysts to form furan-2,5-dicarboxylate (FDCA2−), which is subsequently protonated to produce FDCA. Previously, high yields were achieved on small-scale reactions using caesium furoate and Cs2CO3. In this work, we investigate the carboxylation reaction using alkali furoate/M2CO3 salts containing cation blends and describe reaction conditions that provide high yields on a preparative scale. We show that the carboxylation proceeds efficiently with K+/Cs+ blends that have a high K+ content (up to 4 : 1 K+ : Cs+). Removing H2O, which is a by-product of the reaction, is important for suppressing decomposition pathways. The accumulation of the FDCA2− product inhibits the reaction. Integrating these lessons, we demonstrate the carboxylation of furoate on a 1 mol scale using a fixed-bed flow reactor with 89% isolated yield of pure FDCA upon protonation.
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...