Reductive amination/cyclization of levulinic acid to pyrrolidones versus pyrrolidines by switching the catalyst from AlCl3 to RuCl3 under mild conditions†
Abstract
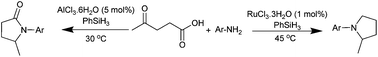
Herein the reductive amination/cyclization of levulinic acid using phenylsilane was presented to selectively produce pyrrolidones versus pyrrolidines under mild conditions by switching the catalyst from AlCl3 to RuCl3. Using AlCl3 as the catalyst, pyrrolidones were solely obtained at room temperature, while RuCl3 as the catalyst selectively afforded pyrrolidines in high yields at 45 °C.


 Please wait while we load your content...
Please wait while we load your content...