Stereoselective, solvent free, highly efficient synthesis of aldo- and keto-N-acylhydrazones applying grindstone chemistry†
Abstract
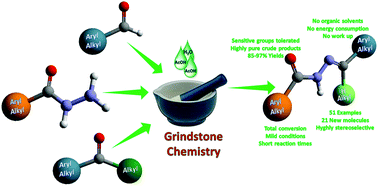
A mild and efficient synthesis of N-acylhydrazones has been developed, applying a simple grindstone procedure, leading to a library of 51 examples exhibiting a broad variety of structural features, 21 of them described for the first time in this paper. This methodology works without any organic solvent under the catalysis of acetic acid at room temperature, promotes the formation of essentially pure crude products, and avoids tedious work-up and the use of harmful solvents, as well as energy consumption. In addition, it leads to the stereoselective formation of N-acylhydrazones, as supported by NMR analysis.


 Please wait while we load your content...
Please wait while we load your content...