A solid acetylene reagent with enhanced reactivity: fluoride-mediated functionalization of alcohols and phenols†
Abstract
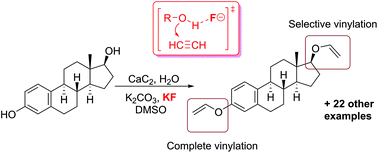
The direct vinylation of an OH group in alcohols and phenols was carried out utilizing a novel CaC2/KF solid acetylene reagent in a simple K2CO3/KOH/DMSO system. The functionalization of a series of hydroxyl-group-containing substrates and the post-modification of biologically active molecules were successfully performed using standard laboratory equipment, providing straightforward access to the corresponding vinyl ethers. The overall process developed involves an atom-economical addition reaction employing only inorganic reagents, which significantly simplifies the reaction set-up and the isolation of products. A mechanistic study revealed a dual role of the F− additive, which both mediates the surface etching/renewal of the calcium carbide particles and activates the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond towards the addition reaction. The development of the fluoride-mediated nucleophilic addition of alcohols eliminates the need for strong bases and may substantially extend the areas of application of this attractive synthetic methodology due to increasing functional group tolerance. As a replacement for dangerous and difficult to handle high-pressure acetylene, we propose the solid reagent CaC2/KF, which is easy to handle, does not require dedicated laboratory equipment and demonstrates enhanced reactivity of the acetylenic triple bond. Theoretical calculations have shown that fluoride-mediated activation of the hydroxyl group towards nucleophilic addition significantly reduces the activation barrier and facilitates the reaction.
C bond towards the addition reaction. The development of the fluoride-mediated nucleophilic addition of alcohols eliminates the need for strong bases and may substantially extend the areas of application of this attractive synthetic methodology due to increasing functional group tolerance. As a replacement for dangerous and difficult to handle high-pressure acetylene, we propose the solid reagent CaC2/KF, which is easy to handle, does not require dedicated laboratory equipment and demonstrates enhanced reactivity of the acetylenic triple bond. Theoretical calculations have shown that fluoride-mediated activation of the hydroxyl group towards nucleophilic addition significantly reduces the activation barrier and facilitates the reaction.


 Please wait while we load your content...
Please wait while we load your content...