Induction of lignin solubility for a series of polar ionic liquids by the addition of a small amount of water†
Abstract
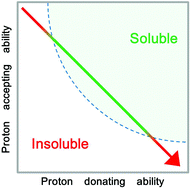
Addition of a small amount of water was found to induce the lignin solubilizing ability in several polar ionic liquids which showed no lignin solubility in the absence of water. Similarly, addition of water was found to enhance lignin solubility in many polar ionic liquids. Though addition of water lowered the proton accepting ability of these ionic liquids, their proton donating ability was found to increase. The lignin dissolution by ionic liquids was newly found to be a function of both the proton accepting ability and proton donating ability of the ionic liquids. Water is a poor solvent for polysaccharides, and water addition has therefore been confirmed to be effective to improve the selective extraction yield of lignin from cedar powder under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...