Towards nitrile-substituted cyclopropanes – a slow-release protocol for safe and scalable applications of diazo acetonitrile†
Abstract
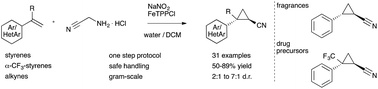
Diazo acetonitrile has long been neglected despite its high value in organic synthesis due to a high risk of explosions. Herein, we report our efforts towards the transient and safe generation of this diazo compound, its applications in iron catalyzed cyclopropanation and cyclopropenation reactions and the gram-scale synthesis of cyclopropyl nitriles.


 Please wait while we load your content...
Please wait while we load your content...