Abstract
An environmentally benign, metal-free synthesis of sulfonyl chlorides and bromides from thiols in the presence of ammonium nitrate, an aqueous solution of HCl and HBr and oxygen as a terminal oxidant was developed. The reactivity of various substituted thiophenols, benzylic-, aliphatic- and heteroaromatic thiols was examined. Ammonium nitrate served as a source of nitrogen oxides (NO/NO2), which are the crucial players in a redox-catalytic cycle. Sulfonyl chlorides and bromides were isolated without extraction and “filtered” over a short pad of silica gel; the use of solvents was greatly reduced in comparison with traditional isolation and purification. A “one-pot” protocol for the conversion of thiol into sulfonamide is also demonstrated. Scale-up experiments on the preparation of sulfonyl chloride and bromide are shown. A possible reaction pathway is discussed.
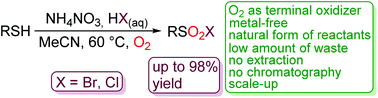


 Please wait while we load your content...
Please wait while we load your content...