Mild-temperature hydrodeoxygenation of vanillin over porous nitrogen-doped carbon black supported nickel nanoparticles†
Abstract
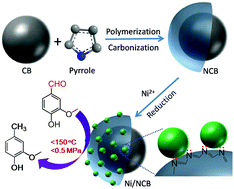
Porous nitrogen-doped carbon black (NCB) was synthesized by facile carbonization of carbon black (CB) coated with polypyrrole (CB@polypyrrole) and used as a support for Ni nanoparticles (NPs). The microstructure, reducibility and crystallinity of the as-synthesized materials were investigated by transmission electron microscopy (TEM), H2-TPR/TPD, X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD). It was found that surface nitrogen species on NCB significantly promote the decomposition of the nickel precursor and the reduction of nickel oxide, and improve the stability of metallic Ni in ambient atmosphere. In the selective hydrodeoxygenation (HDO) of vanillin in the aqueous phase at low hydrogen pressure (0.5 MPa) and mild temperature (<150 °C), Ni/NCB shows much higher activity than N-free catalysts. This is ascribed to the higher reducibility, the lower oxidation state of Ni NPs and the enhanced hydrogen spillover of Ni to the support. Moreover, the Ni/NCB catalyst is relatively cheap and easy to scale-up the production of, thus achieving a low-cost transformation of biomass to bio-oils.


 Please wait while we load your content...
Please wait while we load your content...