Carboxylate-promoted reductive functionalization of CO2 with amines and hydrosilanes under mild conditions†
Abstract
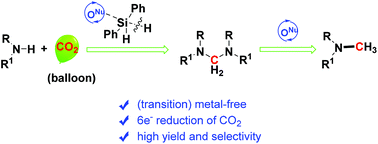
Various oxygen-nucleophiles especially carboxylates, e.g. cesium/tetrabutylammonium carboxylate, were proved to be efficient and selective catalysts for reductive functionalization of CO2 with amines and hydrosilanes to methylamines. Various amines including aromatic and aliphatic, primary and secondary ones were methylated successfully in the presence of diphenylsilane as the reductant under 50 °C and an atmospheric pressure of CO2. Furthermore, a reaction pathway involving CO2 reduction to the C0 species i.e. aminal rather than the formamide as the intermediate was proposed. This protocol represents a transition metal-free and environmentally friendly option for CO2 conversion to useful chemicals via the formation of C–N bonds coupled with six-electron reduction of CO2 to the methanol level under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...