Nitrogen-doped carbon nanotubes encapsulate cobalt nanoparticles as efficient catalysts for aerobic and solvent-free selective oxidation of hydrocarbons†
Abstract
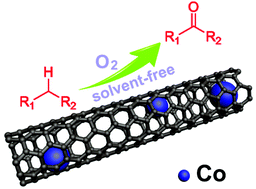
The selective oxidation of hydrocarbons to the corresponding ketones with solvent-free and molecular oxygen as an oxidant is of great importance in academic and industrial fields in view of economy and environment. In this respect, we present the facile synthesis and characterization of excellent catalysts comprising cobalt nanoparticles encapsulated into graphitic nitrogen-doped carbon nanotubes (Co@GCNs) via one-pot pyrolysis of a chelate compound containing citric acid, melamine, and CoCl2·6H2O. The selective oxidation of ethylbenzene under molecular oxygen and solvent-free conditions is employed as a probe reaction to investigate the catalytic performance; the optimized catalyst shows the best conversion (68%) and selectivity for acetophenone (93%). Combination of the catalytic results of the control group and the different characterization methods demonstrates that high catalytic activity is due to the synergistic effect between metallic cobalt and nitrogen-doped carbon nanotubes. Moreover, the catalyst has high catalytic activity for the aerobic and solvent-free oxidation of other arylalkane substrates. The proposed mechanistic study illustrates that the reaction is a free radical reaction progressing through superoxide radical anions (˙O2−).
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...