Dissolution of pyrite and other Fe–S–As minerals using deep eutectic solvents
Abstract
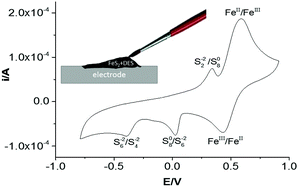
Processing sulfur containing minerals is one of the biggest sources of acute anthropogenic pollution particularly in the form of acid mine drainage. This study attempts to show an innovative method for processing sulfide-based minerals. It is shown that pyrite can be solubilised by both electrochemical oxidation and reduction in a deep eutectic solvent (DES) Ethaline, a mixture of choline chloride and ethylene glycol. A novel method is demonstrated to investigate the redox properties of minerals using a paste made from the mineral powder in a DES. The first bulk electrochemical dissolution of pyrite is shown without the formation of H2S or SO2. It is also shown that the soluble species, including elements such as arsenic, can be recovered electrochemically which could potentially decrease acid mine drainage. The electrochemical properties of other iron–sulfur and iron–arsenic minerals are also presented and compared to those of pyrite.


 Please wait while we load your content...
Please wait while we load your content...