Metal-free catalytic conversion of CO2 and glycerol to glycerol carbonate†
Abstract
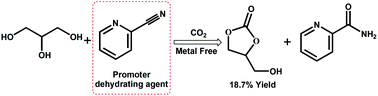
Using CO2 as a carbonyl building block to synthesize valuable chemicals is an important issue in the field of green and sustainable chemistry. Herein, the conversion of CO2 with glycerol to glycerol carbonate was investigated in the presence of 2-cyanopyridine. It was found that 2-cyanopyridine not only acts as the dehydrating agent to break the thermodynamic limit of the reaction, but also activates the carbonyl bond of CO2 to catalyze the present carbonylation as confirmed by FTIR experimental spectra. Moreover, the activation closely depends on the stereo-structure of cyanopyridine, in that 3-cyanopyridine and 4-cyanopyridine do not have such activating function. The theoretical calculation demonstrated that 2-cyanopyridine could bond CO2 through the conjugating nitrogen to form a five-membered ring, thus CO2 is activated and then reacts with glycerol to produce glycerol carbonate. The reaction parameters were examined and evaluated for the reaction catalyzed with 2-cyanopyridine in the absence of any metal catalysts and additives. With increasing CO2 pressure, the yield of glycerol carbonate increases, and under the optimum conditions an 18.7% yield is obtained which is one of the best results reported up to now. The present work provides a new way for activation of CO2.


 Please wait while we load your content...
Please wait while we load your content...