Continuous flow ring-closing metathesis, an environmentally-friendly route to 2,5-dihydro-1H-pyrrole-3-carboxylates†
Abstract
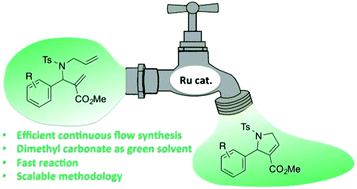
2,5-Dihydro-1H-pyrrole-3-carboxylates are important building blocks for the synthesis of high value pyrroles and pyrroloquinoline derivatives with interesting biological activities. The use of continuous flow allowed us to perform a key synthetic step, namely ruthenium-catalyzed ring-closing metathesis, with a residence time of 1 min at 120 °C. Dimethyl carbonate, a green solvent, was demonstrated for the first time to be an excellent solvent for this reaction in continuous flow. The continuous flow conditions proved to be general and the scale-up of this reaction was not only possible, but also highly efficient. Conversion of 10 grams of diene was realized in 37 minutes under continuous flow, yielding the desired heterocycle in 91% yield.
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...