Phenolic acetals from lignins of varying compositions via iron(iii) triflate catalysed depolymerisation†
Abstract
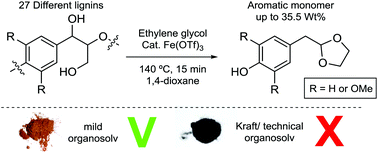
Lignin is a highly abundant, renewable aromatic polymer that can potentially be obtained in large quantities from lignocellulosic biorefineries. Thus the valorisation of this renewable resource by the production of aromatic chemicals would be highly desirable and is especially important for achieving high yields of these products. In this regard, not only the catalytic method used should be highly selective, but also we must better understand the possible correlations between the structure of the lignin used and the yield of useful products. Here, we demonstrate that lignins obtained from a range of different biomass sources and pretreatment methods can be successfully depolymerized using iron(III) triflate in the presence of ethylene glycol to give p-(1,3-dioxolan-2-yl)methyl substituted phenols. 27 lignins, obtained from 13 different pretreatment methods, were examined in this study. A combined yield of up to 35.5 wt% of acetal products was obtained from a β-aryl ether rich organosolv lignin and the best yield of a single component (16.5 wt%) was achieved starting from pine lignin. Much lower yields were obtained from technical lignins which were low in β-aryl ether content, whilst a range of organosolv lignins of intermediate β-aryl ether content gave intermediate yields of acetal products. Overall, correlations were found between the product distributions and yields and structural data of the parent lignins obtained from 2D HSQC NMR analysis.
- This article is part of the themed collections: 2017 Green Chemistry Hot Articles and Green Chemistry 2017 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...