Antimony(v) catalyzed acetalisation of aldehydes: an efficient, solvent-free, and recyclable process†
Abstract
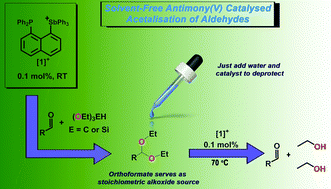
A highly selective, solvent-free process for the acetalisation of aldehydes was achieved by the use of a readily accessible antimony(V) catalyst which we previously prepared in our lab as a tetraarylstibonium triflate salt ([1][OTf]). High yields of the acetals were achieved in the presence of stoichimetric amounts of either triethoxymethane or triethoxysilane. It was found that triethoxymethane reactions required longer time to reach completion when compared to triethoxysilane reactions which were completed upon mixing of the reagents. The products can be easily separated from the catalyst by distillation which enabled further use of [1][OTf] in additional calytic reactions (up to 6 cycles). Moreover, [1]+ also catalyzed the deprotection of the acetals into their corresponding aldehydes using only water as a solvent.


 Please wait while we load your content...
Please wait while we load your content...