Mechanism of C–C bond formation in the electrocatalytic reduction of CO2 to acetic acid. A challenging reaction to use renewable energy with chemistry
Abstract
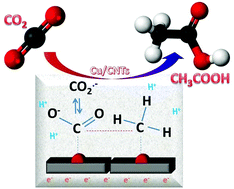
Copper nanoparticles on carbon nanotubes are used in the reduction of CO2 to acetic acid (with simultaneous water electrolysis) in a flow electrocatalytic reactor operating at room temperature and atmospheric pressure. A turnover frequency of about 7000 h−1 and a carbon-based Faradaic selectivity to acetic acid of about 56% were observed, indicating potential interest in this approach for using renewable energy. The only other products of reaction detected were formic acid and methanol (the latter in some cases), besides H2. The reaction mechanism, particularly the critical step of C–C bond formation, was studied by comparing the reactivity in tests with CO2 or CO, where formic acid or formaldehyde where initially added. The results indicate the need for having dissolved CO2 to form acetic acid, likely via the reaction of CO2˙− with surface adsorbed –CH3 like species. The pathway towards formic acid is instead different from the route of the formation of acetic acid.
- This article is part of the themed collection: Harvesting Renewable Energy with Chemistry


 Please wait while we load your content...
Please wait while we load your content...