Aqueous phase homogeneous formic acid disproportionation into methanol†
Abstract
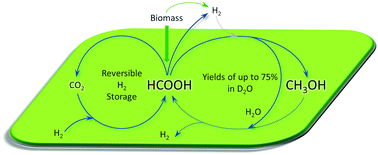
The catalytic activity of a homogeneous iridium complex in formic acid disproportionation into methanol was explored. Formic acid reduction to methanol proceeds efficiently in aqueous media with the methanol yield depending on the nature of the solvent, substrate concentration, applied H2 pressure and reaction temperature. The methanol yield peaked at 75% when D2O was used as a solvent at 50 °C. Increasing the reaction temperature to 80 °C and doubling the substrate concentration led to an improved methanol concentration, even though the corresponding yield dropped to 59%. Initial H2 pressures further enhanced methanol formation, affording a highly concentrated 9.8 m methanol solution or a TON of 1260 upon in situ catalyst recycling under aerobic conditions. No catalyst deactivation was observed for five cycles.
- This article is part of the themed collection: Harvesting Renewable Energy with Chemistry


 Please wait while we load your content...
Please wait while we load your content...