Metal-catalyzed reductive deamination of glutamic acid to bio-based dimethyl glutarate and methylamines†
Abstract
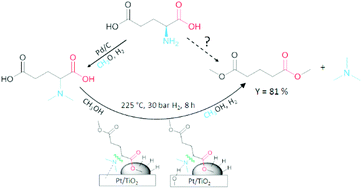
Glutamic acid is a promising renewable platform molecule which is abundantly available in biomass waste streams; it is also efficiently manufactured by fermentation. Here we report the reductive deamination of glutamic acid to bio-based dimethyl glutarate and methylamines. In order to recycle nitrogen in an industrially relevant co-product, glutamic acid was modified to N,N-dimethylglutamic acid by a mild reductive alkylation with Pd/C. Subsequently, selective C–N hydrogenolysis in methanol resulted in dimethyl glutarate and trimethylamine. A wide screening of transition metals (Pt, Pd, Rh and Ru) immobilized on various supports showed that the highest yields of dimethyl glutarate were obtained with Pt/TiO2. An FTIR study and kinetic experiments on metal-loaded and unloaded supports demonstrate that the interplay between the metal and the moderate acidity of the support results in the excellent C–N hydrogenolysis activity and selectivity. Finally, reaction parameter optimization resulted in 81% yield of dimethyl glutarate with 1 wt% Pt/TiO2 at 225 °C, 30 bar H2 after 8 h.
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...