An alternative approach towards poly-ε-caprolactone through a chemoenzymatic synthesis: combined hydrogenation, bio-oxidations and polymerization without the isolation of intermediates†
Abstract
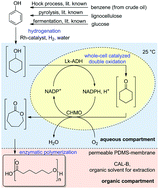
A novel synthetic route towards the polymer poly-ε-caprolactone based on a chemoenzymatic reaction sequence was developed. Initial hydrogenation of phenol to cyclohexanol gave a crude product, which was directly used without work-up for a subsequent biocatalytic double oxidation towards ε-caprolactone by means of an alcohol dehydrogenase and a monooxygenase. In order to overcome product inhibition effects, an in situ-product removal strategy via extraction of ε-caprolactone from an aqueous reaction medium with an organic solvent in the presence of a permeable polydimethylsiloxane membrane was applied. Furthermore, this in situ-product removal was combined with lipase-catalyzed polymerization in the organic phase at 25 °C. The obtained crude product contained a polymer fraction with a degree of polymerization comparable to commercial poly-ε-caprolactone.
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles

 Please wait while we load your content...
Please wait while we load your content...