Hierarchically nanostructured MnCo2O4 as active catalysts for the synthesis of N-benzylideneaniline from benzyl alcohol and aniline†
Abstract
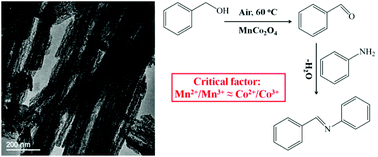
A facile and scaled-up synthesis route to hierarchically nanostructured transition metal oxides with desirable properties is of great practical importance because of their excellent performance as heterogeneous catalysts in organic synthesis. In this work, hierarchically nanostructured MnCo2O4 nanorods with multi-nanopores have been prepared by a facile co-precipitation method using oxalic acid as a precipitant and through their consequent removal by calcination. When evaluated as catalysts for the synthesis of N-benzylideneaniline from benzyl alcohol and aniline, the as-prepared hierarchically nanostructured MnCo2O4-500 nanorods possessed high conversion (90.9%) of benzyl alcohol and selectivity (95.4%) of N-benzylideneaniline at 60 °C even under air for 15 h, which can be attributed to the appropriate and similar ratios of Mn2+/Mn3+ (1.36 : 1) and Co2+/Co3+ (1.35 : 1) with excellent synergistic effects. The proposed mechanism reveals that the benzyl alcohol is initially dehydrogenated to benzaldehyde which then reacts with another molecule of aniline to form N-benzylideneaniline. The MnCo2O4-500 nanorods can also be easily recycled without significant loss in catalytic activity for at least 4 cycles. Our findings could provide some guidance on the design of nanostructured spinel-type metal oxide catalysts with better synergistic effects in organic synthesis.

 Please wait while we load your content...
Please wait while we load your content...