Enantioselective chemoenzymatic synthesis of a key segment of neuronal nitric oxide synthase inhibitors and several related 3-aminopyridinylmethyl-4-hydroxypyrrolidines†
Abstract
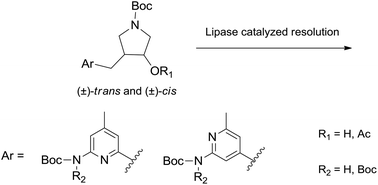
The lipase catalyzed resolution of several cis and trans 3-aminopyridinylmethyl-4-hydroxypyrrolidines by acylation or hydrolysis has been studied. Some of these pryrrolidines are precursors in the synthesis of selective nNOS inhibitors; for this purpose, the best result is obtained in the resolution of (±)-trans-5via a CAL-A catalysed acylation. The high enantioselectivity of this process allows one to obtain in high yield the optically pure (3S,4R)-configured product that has the correct configuration to complete the synthesis of nNOS inhibitors. The study described here highlights the effect of structural variations remote from the stereogenic centers of the molecule on the reactivity and enantioselectivity of the enzymatic processes.
- This article is part of the themed collection: Enzyme catalysis in organic synthesis

 Please wait while we load your content...
Please wait while we load your content...