Catalyst-free synthesis of fused 1,2,3-triazole and isoindoline derivatives via an intramolecular azide–alkene cascade reaction†
Abstract
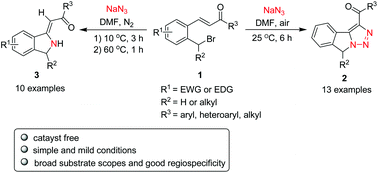
Benzyl bromides bearing an ortho-substituted α,β-unsaturated ketone moiety are found to be promising precursors for the synthesis of fused 1,2,3-triazole and isoindoline derivatives via an intramolecular azide–alkene cascade reaction under catalyst free conditions. Fused 1,2,3-triazole derivatives 2 were synthesized in excellent yields by treating compounds 1 with sodium azide in DMF at room temperature, whereas isoindoline derivatives 3 were produced in moderate to good yields only by slight modification of the reaction conditions. Both reactions call for simple and mild conditions, display broad substrate scopes and result in good regiospecificity.


 Please wait while we load your content...
Please wait while we load your content...