The mechanism of reduced IgG/IgE-binding of β-lactoglobulin by pulsed electric field pretreatment combined with glycation revealed by ECD/FTICR-MS†
Abstract
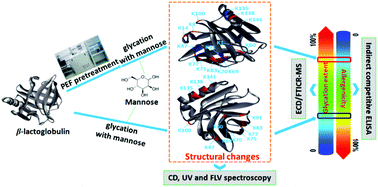
Bovine β-lactoglobulin (β-Lg) is a major allergen existing in milk and causes about 90% of IgE-mediated cow's milk allergies. Previous studies showed that pulsed electric field (PEF) treatment could partially unfold the protein, which may contribute to the improvement of protein glycation. In this study, the effect of PEF pretreatment combined with glycation on the IgG/IgE-binding ability and the structure of β-Lg was investigated. The result showed that PEF pretreatment combined with glycation significantly reduced the IgG and IgE binding abilities, which was attributed to the changes of secondary and tertiary structure and the increase in glycation sites and degree of substitution per peptide (DSP) value determined by electron capture dissociation Fourier transform ion cyclotron resonance mass spectrometry (ECD/FTICR-MS). Unexpectedly, glycation sites (K47, K91 and K135) added by two mannose molecules were identified in glycated β-Lg with PEF pretreatment. Moreover, the results indicated that PEF pretreatment at 25 kV cm−1 for 60 μs promoted the reduction of IgG/IgE-binding capacity by increasing the glycation degree of β-Lg, whereas single PEF treatment under the same conditions markedly enhanced the IgG/IgE-binding ability by partially unfolding the structure of β-Lg. The results suggested that ECD/FTICR-MS could help us to understand the mechanism of reduction in the IgG/IgE-binding of β-Lg by structural characterization at the molecular level. Therefore, PEF pretreatment combined with glycation may provide an alternative method for β-Lg desensitization.


 Please wait while we load your content...
Please wait while we load your content...