Formation of crystalline nanoparticles by iron binding to pentapeptide (Asp-His-Thr-Lys-Glu) from egg white hydrolysates
Abstract
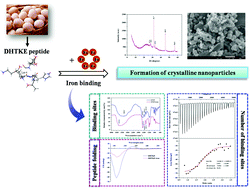
A novel peptide from egg white, Asp-His-Thr-Lys-Glu (DHTKE), contains specific amino acids associated with iron binding. The present study aims to better understand the molecular basis of interactions between the DHTKE peptide and iron ions. The ultraviolet-visible and fluorescence spectra indicate an interaction between the DHTKE peptide and iron ions, which leads to the formation of a DHTKE–iron complex. Notably, Asp, Glu, His, and Lys in the DHTKE peptide play crucial roles in the formation of the DHTKE–iron complex, and the iron-binding site of the DHTKE peptide corresponds primarily to the amide and carboxyl groups. The DHTKE peptide can bind iron ions in a 1 : 2 ratio with a binding constant of 1.312 × 105 M−1. Moreover, the DHTKE–iron complex belongs to thermodynamically stable nanoparticles that are present in the crystalline structure, which might be attributed to peptide folding induced by iron binding. Meanwhile, the DHTKE–iron complex exhibits a relatively high iron-releasing percentage and exerts excellent solubility in the human gastrointestinal tract in vitro. This suggests a potential application of peptides containing Asp, Glu, His, or Lys residues as potential iron supplements.


 Please wait while we load your content...
Please wait while we load your content...