An inhibition mechanism of dihydromyricetin on tyrosinase and the joint effects of vitamins B6, D3 or E
Abstract
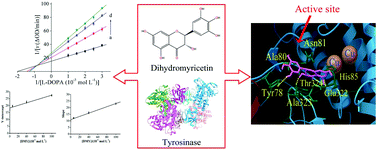
Dihydromyricetin (DMY), a natural flavonoid, was found to effectively inhibit tyrosinase activity in a mixed-type manner with an IC50 value of (3.66 ± 0.14) × 10−5 mol L−1. DMY combined with the dietary vitamin D3 at lower concentrations exhibited a synergistic effect on the inhibition of tyrosinase. The formation of a DMY–tyrosinase complex led to fluorescence quenching and conformational changes of tyrosinase, which was driven mainly by hydrophobic interactions and hydrogen bonds. The molecular simulation further found that DMY inserted into the active pocket of tyrosinase interacted with amino acid residues Tyr78, His85, and Ala323, occupying the catalytic center of tyrosinase to hinder entrance of the substrate, leading to the inhibition of tyrosinase. This study may provide a scientific foundation for screening effective tyrosinase inhibitors.


 Please wait while we load your content...
Please wait while we load your content...