Changes of structure and IgE binding capacity of shrimp (Metapenaeus ensis) tropomyosin followed by acrolein treatment†
Abstract
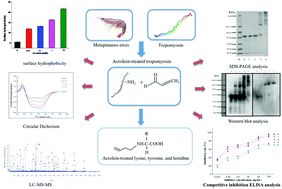
Tropomyosin, a major allergen of shrimp, presents high stability to various conditions owing to the high content of α-helix. Chemical modification of tropomyosin can induce structural changes and affects IgE binding capacity. Since lipid peroxidant such as acrolein commonly exists during shrimp preservation, it is necessary to evaluate its influence on tropomyosin structure and IgE binding capacity. Western blot and inhibition enzyme-linked immunosorbent assay were used to illustrate IgE binding capacity, while structural changes were investigated by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and fluorescence spectroscopy. Results revealed that IgE binding capacity reduced and the secondary structure denatured following treatment with increasing concentration of acrolein. Furthermore, the identification of LC-MS/MS indicated that the free amines of lysine, tyrosine, and histidine residues were altered by acrolein. These amino acid modifications resulted in the collapse of epitopes, as well as structural changes responsible for the decreased IgE binding capacity of shrimp tropomyosin.


 Please wait while we load your content...
Please wait while we load your content...