Optical backbone-sidechain charge transfer transitions in proteins sensitive to secondary structure and modifications†
Abstract
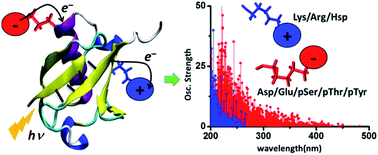
The absorption of light by proteins can induce charge transfer (CT) transitions in the UV-visible range of the electromagnetic spectrum. Metal–ligand complexes or active site prosthetic groups which absorb in the visible region exhibit prominent CT transitions. Furthermore, the protein backbone also exhibits CT transitions in the far UV range. In this manuscript, we present a detailed computational study of new near UV-visible CT transitions that involve amino acids with charged side chains. Specifically, using time dependent density functional theory calculations, we examine the absorption spectra of naturally charged amino acids (Lys, Glu, Arg, Asp and His), extracted from solution phase protein structures generated by classical molecular dynamics simulations, and phosphorylated amino acids (Tyr, Thr and Ser) from experimentally determined protein structures. We show that amino acids with charged sidechains present a directed electronic donor–bridge–acceptor paradigm, with the lowest energy optical excitations demonstrating peptide backbone-sidechain charge separations. The UV-visible spectral range of the backbone-sidechain CT transitions is determined by the chemical nature of the donor, bridge and acceptor groups within each amino acid, amino acid conformation and the protein secondary structure where the amino acids are located. Photoinduced CT occurs in opposite directions for the anionic and cationic amino acids along the ground state dipole moment vector for the chromophores. We find that photoinduced charge separation is more facile for the anionic amino acids (Asp, Glu, pSer, pThr and pTyr) relative to that for the cationic amino acids (Lys, Arg and Hsp). Our results provide a foundation for the development of spectroscopic markers based on the recently proposed Protein Charge Transfer Spectra (ProCharTS) which are relevant for the study of DNA-binding or intrinsically disordered proteins that are rich in charged amino acids.
- This article is part of the themed collections: New Frontiers in Indian Research, New Frontiers in Indian Research and Photoinduced Processes in Nucleic Acids and Proteins


 Please wait while we load your content...
Please wait while we load your content...