Optically sensing phospholipid induced coil–helix transitions in the phosphoinositide-binding motif of gelsolin†
Abstract
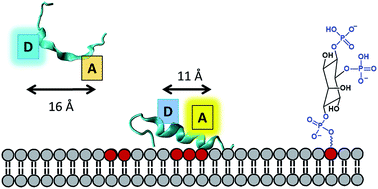
We present a systematic experimental and computational study of phospholipid induced peptide coil–helix transitions which are relevant in the context of proteins mediating cytoskeletal rearrangement via membrane binding. We developed a sensitive Förster resonance energy transfer (FRET) based assay to address whether coil–helix transitions in phospholipid binding motifs of actin-binding proteins can be induced by physiologically-relevant concentrations (1–20 μM) of phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) phospholipids. Based on inter-residue distance constraints obtained from Molecular Dynamics (MD) simulations of a 20 residue peptide (Gel 150–169) from the actin-severing protein gelsolin, we synthetized and labeled the peptide with a tryptophan donor and IAEDANS acceptor pair. Upon addition of PI(4,5)P2 micelles and mixed vesicles containing PI(4,5)P2 and phosphatidylcholine to the peptide, we observed a decrease in the tryptophan emission intensity with increasing concentrations of PI(4,5)P2. The IAEDANS emission spectra showed a more complex profile exhibiting a blue shift of the emission peak and non-monotonic changes in the intensity profile with increasing concentrations of PI(4,5)P2. We showed that the IAEDANS acceptor emission response is a result of both intrinsic polarity sensitivity of the acceptor in the vicinity of the membrane surface and fluorescence energy transfer from the donor. Importantly, the fluorescence lifetime of the donor (tryptophan) showed a monotonous decrease with increasing mol% of PI(4,5)P2 from 1.13 ± 0.10 ns in the absence of phospholipids to 0.25 ± 0.03 ns in the presence of 100% PI(4,5)P2 micelles. We also showed a concomitant increase in FRET efficiency with increasing PI(4,5)P2 levels indicating a PI(4,5)P2 concentration dependent coil–helix transition. Our studies demonstrate that membrane PI(4,5)P2 concentrations as low as 2.5–5 μM can trigger helix–coil conformational changes in gelsolin relevant for triggering regulatory processes in the cell.
- This article is part of the themed collections: New Frontiers in Indian Research, New Frontiers in Indian Research and Photoinduced Processes in Nucleic Acids and Proteins


 Please wait while we load your content...
Please wait while we load your content...