Photochemical relaxation pathways of S6-methylthioinosine and O6-methylguanosine in solution†
Abstract
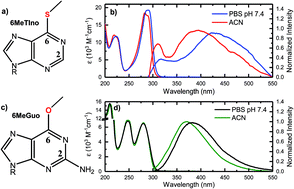
S6-Methylthioinosine and O6-methylguanosine are byproducts resulting from the enzymatic reactions of sulfur-substituted prodrugs in cells and from the interaction of alkylating agents with cellular DNA, respectively. Their photochemistry has not been investigated, and it is currently unknown whether light absorption by these byproducts may pose any threat to the cell. In this contribution, their photoinduced processes upon absorption of UVB radiation are reported using broadband transient absorption spectroscopy. Plausible electronic relaxation mechanisms are proposed for both biological molecules, which are supported by steady-state absorption and emission measurements, and by singlet and triplet vertical excitation energies performed on a large subset of ground-state optimized conformational isomers in solution. The results are compared to the body of knowledge gathered in the scientific literature about the light-induced processes in the sulfur-substituted and canonical purine monomers. In particular, it is shown that S6-methylation decreases the rate to populate the lowest-energy triplet state and blueshifts the ground-state absorption spectrum compared to those for the sulfur-substituted prodrugs and for the 6-thioguanosine metabolite. Similarly, O6-methylation decreases the rate of internal conversion to the ground state observed in the guanine monomers by more than 10-fold in acetonitrile and 40-fold in aqueous solution, while it redshifts the ground-state absorption spectrum. Collectively, this investigation provides relevant new insights about the relationship between structural modifications of the purine chromophore and the electronic relaxation mechanisms in this important group of biological molecules.
- This article is part of the themed collection: Photoinduced Processes in Nucleic Acids and Proteins


 Please wait while we load your content...
Please wait while we load your content...