Time-resolved SERS study of the oxygen reduction reaction in ionic liquid electrolytes for non-aqueous lithium–oxygen cells
Abstract
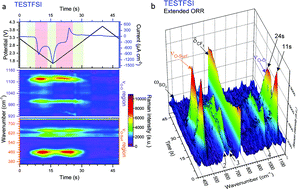
Superoxide (O2˙−) is the key intermediate formed during oxygen reduction in non-aqueous electrolytes. One significant obstacle towards the realisation of a practical lithium–oxygen (Li–O2) battery is electrolyte instability in the presence of radical oxides, principally superoxide. Here we use the Raman active bands of O2˙− as a diagnostic molecule for probing the influence of the electrolyte on reaction processes and intermediaries at the electrode surface. In situ surface enhanced Raman studies of the interface at a roughened Au electrode with controlled and dynamic surface potentials were performed in two ionic liquids with differing properties: 1-butyl-1-methyl-azepenium bis(trifluoromethanesulfonyl)imide (Aze14TFSI), which has a large/soft cation, and triethylsulfonium bis(trifluoromethanesulfonyl)imide (TESTFSI), which has a relatively small/hard and e− accepting cation. The counter-cation and potential were seen to significantly influence the radical nature, or Lewis basicity of O2˙−. The analysis of peak intensities and Stark shifts in O2˙− related spectral bands allowed for key information on its character and electrolyte interactions to be elucidated. Time-resolved studies of dynamic surface potentials permitted real time observation of the flux and reorientation of ions at the electrode/electrolyte interface.
- This article is part of the themed collection: Ionic liquids: from fundamental properties to practical applications


 Please wait while we load your content...
Please wait while we load your content...