Exploring the bulk-phase structure of ionic liquid mixtures using small-angle neutron scattering†
Abstract
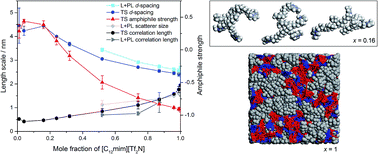
Small-angle neutron scattering experiments, supported by molecular dynamics simulations, have been performed on a range of compositions of the [C2mim]1−x[C12mim]x[Tf2N] ionic liquid mixture system. Isotopic contrast variation, through selective deuteration of both cations, has been used to assist in fitting the data to different scattering models. These data, and subsequent fitting, show that the structure of the ionic liquid mixtures changes substantially as a function of composition. Mixtures where x < 0.32 are dominated by aggregates of amphiphilic [C12mim]+ ions in the relatively polar [C2mim][Tf2N] solvent. Compositions where x > 0.32 can be described as bicontinuous, containing networks of both polar and non-polar domains, where the C12 chains of the [C12mim]+ ions percolate through the system to form a continuous non-polar sub-phase. Temperature-dependent scattering experiments suggest that there is relatively little change in bulk structure in these liquids between 20 and 60 °C. The presence of water, however, does influence some aspects of the liquid structure in a composition that is rich in [C2mim][Tf2N] (where x = 0.24).
- This article is part of the themed collection: Ionic liquids: from fundamental properties to practical applications


 Please wait while we load your content...
Please wait while we load your content...