The Au(111)/IL interfacial nanostructure in the presence of precursors and its influence on the electrodeposition process
Abstract
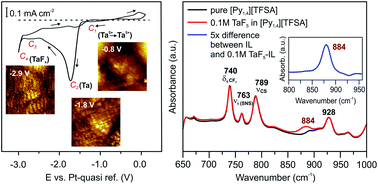
Ionic liquids have attracted significant interest as electrolytes for the electrodeposition of metals and semiconductors, but the details of the deposition processes are not yet well understood. In this paper, we give an overview of how the addition of various precursors (TaF5, SiCl4, and GaCl3) affects the solid/IL interfacial structure. In situ Atomic Force Microscopy (AFM) and vibrational spectroscopy have been employed to study the changes of the Au(111)/IL interface and in the electrolytes, respectively. Ionic liquids with the 1-butyl-1-methylpyrrolidinium ([Py1,4]+) cation and bis(trifluoromethylsulfonyl)amide ([TFSA]−), trifluoromethylsulfonate ([TfO]−) and tris(pentafluoroethyl)trifluorophosphate ([FAP]−) as anions were chosen for this purpose. In situ AFM force–distance measurements reveal that both the anion of the IL and the solutes (TaF5 or GaCl3) influence the Electrical Double Layer (EDL) structure of the Au(111)/IL interface, which can affect the deposition process of Ta and the morphology of the Ga electrodeposits, respectively. Furthermore, the concentration of the precursor can significantly alter the Au(111)/[Py1,4][FAP]–SiCl4 interfacial structure wherein the presence of 0.25 M SiCl4 a double layer structure forms that facilitates Si deposition. This study may provide some critical insights into the structure of the electrode/IL interface for specific applications.
- This article is part of the themed collection: Ionic liquids: from fundamental properties to practical applications


 Please wait while we load your content...
Please wait while we load your content...