Investigation of pH-dependent phosphate removal from wastewaters by membrane capacitive deionization (MCDI)†
Abstract
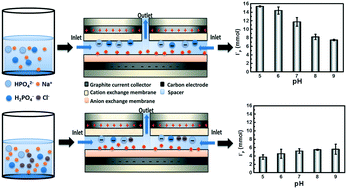
Membrane capacitive deionization (MCDI) is a promising technology for the removal of charged species from aqueous feed streams. In this study, the effects of initial pH on the removal of phosphate (P) species by MCDI were investigated. Two operational modes (constant voltage (CV) and constant current (CC)) were adopted with both the efficacy of P removal and the energy consumption between CV and CC modes compared at different initial pH values. Results indicated that, for feed water only containing phosphate species, the optimized P removal performance was achieved at pH 5.0–6.0 where the monovalent P species (i.e., H2PO4−) dominated in CC mode more energy-efficiently in terms of P removal than in CV mode. The competitive electrosorption between Cl− and P over a range of initial pH values was examined in CC mode with results indicating that at pH 5.0 and 6.0, where the monovalent P species (i.e., H2PO4−) dominated, Cl− was preferentially electrosorbed, presumably because of its smaller hydrated radius, while under more alkaline conditions (pH 8.0 and 9.0), divalent P species (i.e., HPO42−) were more selectively removed due to their charge. A Nernst–Planck equation, in which the ion flux ratio was expressed as a function of diffusion coefficient and charge, provides a good description of the ion selectivity between Cl− and P when the initial concentration of these two ions are equal.


 Please wait while we load your content...
Please wait while we load your content...