Chemodynamics and bioavailability of metal ion complexes with nanoparticles in aqueous media†
Abstract
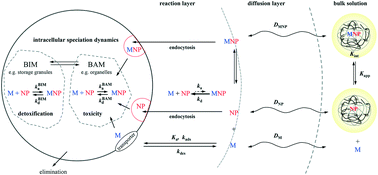
Nanoparticles (NPs) exhibit unique reactivity features that stem from the spatial confinement of their reactive sites to the particle body, which typically carries electric charges. Accordingly, association of ions and molecules with NPs takes place in a local environment that may be very different from that prevailing in the bulk aqueous medium. We present a critical overview of a conceptual framework that describes the dynamic features of metal ion, M, association with different types of NPs, i.e. impermeable (hard, 2D), core–shell, and permeable (soft, 3D). The interpretation identifies the crucial role played by the particulate electric field, and elucidates the factors that determine which step in the overall association/dissociation process is the rate-limiting one. The scope encompasses delineation of the distribution of NP-associated metals, M-NP, over various intraparticulate forms, as well as description of the influence of the intraparticulate spatial distribution of reactants on the complex formation/dissociation kinetics. The connection between the chemodynamic features of M-NP entities at the intraparticulate level and their reactivity at the macroscopic scale is elaborated. These relationships are used to derive analytical expressions for the lability of M-NP entities at reactive interfaces, such as sensors and organisms. Such knowledge is required to make mechanistic links with bioavailability and ensuing toxicity. The interpretation includes formulation of the operational reaction layer at the macroscopic interface and the significance of partial size exclusion of the NP body therefrom. The concepts are illustrated by confrontation with sets of experimental data for different types of natural and engineered NPs.


 Please wait while we load your content...
Please wait while we load your content...