The short-term reduction of uranium by nanoscale zero-valent iron (nZVI): role of oxide shell, reduction mechanism and the formation of U(v)-carbonate phases†
Abstract
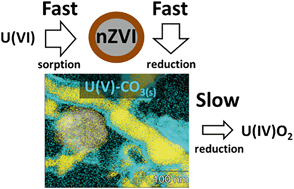
Nanoscale zero-valent iron (nZVI) is a potential remediation agent for uranium-contaminated groundwaters, however, a complete mechanistic understanding of the processes that lead to uranium immobilization has yet to be achieved. In this study, the short-term anoxic reaction of U(VI) with fresh, (anoxic) aged and corroded nZVI particles was investigated under aqueous conditions conducive to the formation of thermodynamically stable U(VI)–Ca–CO3 ternary aqueous complexes. The first stage of the reaction between U(VI) and nZVI was assigned to sorption processes with the formation of surface U(VI)-carbonate complexes. Aged nZVI removed U(VI) faster than either fresh or corroded nZVI and it is hypothesized that U reduction initially occurs through the transfer of one electron from Fe(II) in the nZVI surface oxide layer. Evidence for reduction to U(V) was obtained through X-ray photoelectron spectroscopy and by determination of U–O bond distances of ∼2.05 Å and 2.27 Å, using U LIII-edge X-ray absorption spectroscopy, which are similar to those observed for the U(V) site in the mixed U(V)/U(VI) carbonate mineral wyartite. Scanning transmission electron microscopy also demonstrated that U was present as a nanoparticulate phase after one day of reaction, rather than a surface complex. Further reduction to U(IV), as observed in previous studies, would appear to be rate-limiting and coincident with the transformation of this meta-stable U-carbonate phase to uraninite (UO2).
- This article is part of the themed collection: RACI100: Celebrating Australian Chemistry


 Please wait while we load your content...
Please wait while we load your content...