Removal of SO2 on a nanoporous photoelectrode with simultaneous H2 production†
Abstract
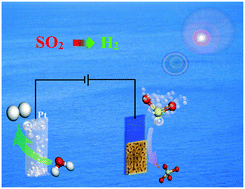
The oxidation process of SO32− to final SO42− is commonly required in existing desulphurization technologies. The resulting SO32− is usually purged with air for oxidation before disposal. The energy of SO32− to SO42− is wasted in this process. Herein, we report simultaneous H2 production with the removal of poisonous SO2via a photoelectrochemical (PEC) water splitting process based on a nanoporous BiVO4 photoanode. The enhancement of H2 production is attributed to the lower activation energy and faster kinetics of SO32− oxidation compared with direct oxidation of water on the photoanode. The enhancement factor is significantly higher on the nanoporous film than on the non-porous film. The removal rate of SO2 is higher than 97%, and the absorbed SO2 is used to produce hydrogen. A H2 evolution rate of 29.8 μmol h−1 cm−2 on the nanoporous BiVO4, with a high Faradaic efficiency, is obtained with removal of SO2, which is much faster than that without SO2 removal (0.22 μmol h−1 cm−2). This work provides a new method for SO2 removal with simultaneous H2 production. The proposed method can be used either in processes involving NaOH as a desulfurization absorber, or combined with existing desulfurization technologies, such as the ammonia-based desulfurization method.


 Please wait while we load your content...
Please wait while we load your content...